A Boolean Model of Microglial Phenotype Transitions Under Acute and Chronic Inflammation
Class of 2020

Biography
- Education
- Thang Long high school for the gifted, Da Lat city, Vietnam
- The College of Wooster, BA in Neurobiology, 2020
- The College of Wooster, minor in Environmental Studies, 2020
- Professional experience
- Sustainable Agriculture Program Intern, Centre for Sustainable Rural Development, Hanoi, Vietnam
- Ambassador, Office of International Student Affairs, College of Wooster
- Health Coach, Wooster Community Hospital
- Undergraduate Research Assistant, Chemistry, College of Wooster
- Undergraduate Research Assistant, Psychology, College of Wooster
- Student Access Assistant at Andrews Library, College of Wooster
- Peer Educator, College of Wooster
Webpage: https://www.linkedin.com/in/bao-nguyen013
IS Thesis Abstract
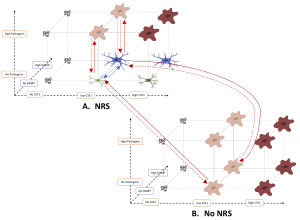
Infection or damage to neurons can trigger the activation of a specialized class of brain-resident immune cell, microglia, which clear infection and help tissue repair. The persistence of microglial activation beyond an acute infection response contributes to many age-related diseases like Alzheimer’s and Parkinson’s. Depending on the nature of neuronal insults, microglia take on an M1 phenotype and secrete cytotoxic mediators, or an M2 phenotype, in which they assist in neuroprotection. Current treatments of neurodegenerative diseases focus on reducing the pro-inflammatory effects caused by the prolonged M1 phenotype. Fewer interventions rely on M2 microglia’s ability to promote tissue repair and wound healing. Here we propose a Boolean regulatory network model that shows the transitions of M1 to M2 signaling and the reverse under acute and chronic inflammatory conditions. Our model offers testable predictions related to environmental factors and specific pathways responsible for microglial morphological remodeling. The results point out the critical role of neuron-microglia crosstalk in regulating microglial activation. Our model broadens the understanding of microglial plasticity in response to different inflammatory signals, which could help diversify therapeutic strategies for neurodegeneration.