Modeling the Impact of Cellular Senescence on Wound Healing in Lung Alveolar Epithelial Tissue
Class of 2025
Biography

- Education
- Qingdao Daewon High School, Shandong, China, 2019
- The College of Wooster, BA in Biology, 2025
- The College of Wooster, Minor in Mathematics and Computer Science, 2025
- Professional experience
- University of Pittsburgh, Department of Computational and Systems Biology, Research Assistant
- APEX Fellowship for Research Assistant Internship
- The College of Wooster, Biology Department, Research Assistant
- Pi Mu Epsilon (National Mathematics Honor Society)
IS Thesis Abstract
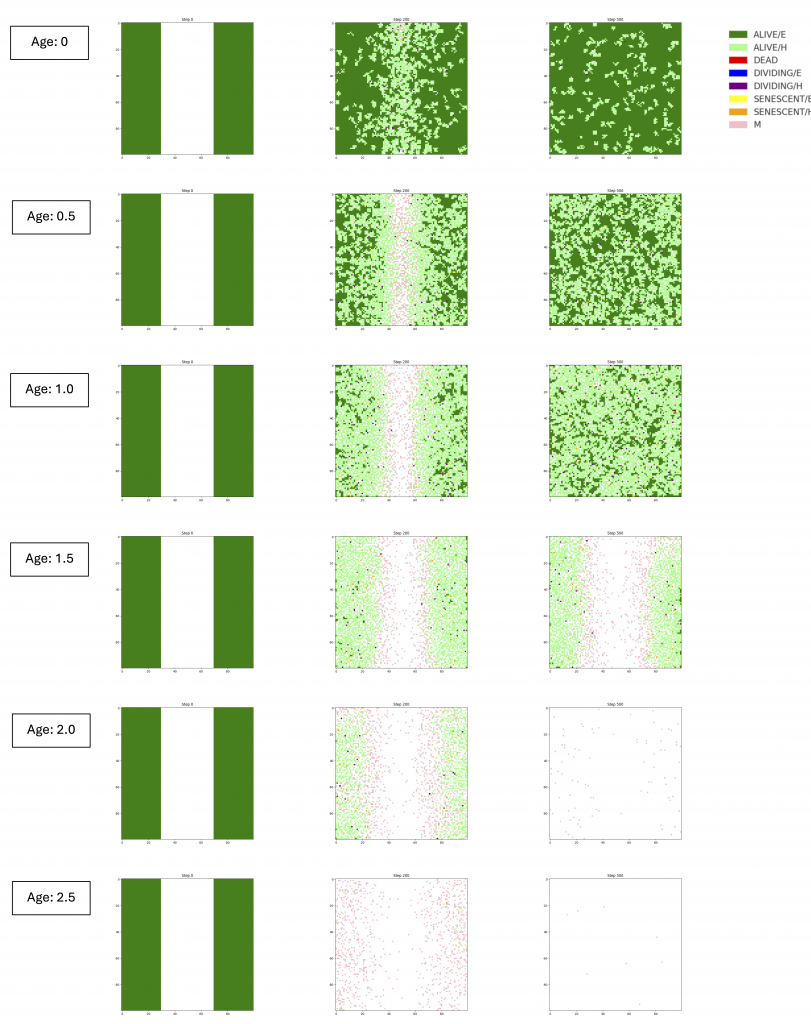
Aging is a leading cause of serious diseases, such as IPF, cardiovascular, and neurodegenerative diseases. Aging involves twelve key factors, most of which are interconnected and can drive one another. Cellular senescence is one of twelve hallmarks of aging. Prolonged cellular senescence can lead to chronic inflammation, tumor development, immune deficit, and stem cell exhaustion, ultimately resulting in tissue dysfunction. However, the dynamics of senescent cell occurrence and removal within a population remain poorly understood. This study models wound healing in lung alveolar tissue, with a focus on the role of cellular senescence dynamics. Wound healing model was expected to reproduce tissue deterioration over time with increasing cellular senescence. Using the Python programming language and its scientific libraries, individual cells, their cell types, and the logic behind cell fate decisions are simulated on a two-dimensional grid. The simulation of wound healing process generated data on division and migration counts, wound area, wound closure step, and average permeability. Four wound healing models were developed, each with distinct features: proliferation-based wound healing model, an EMT-incorporated model, density-directed migration model, and a model integrating senescent cell dynamics. In the absence of molecular dynamics, cell population behavior alone demonstrated a collective migration of cells, delay in wound closure and rise in permeability due to cellular senescence. The fourth model integrating senescent cell dynamics demonstrated tissue deterioration during wound healing as age parameter which determines senescence probability increases, revealing age-related thresholds beyond which tissue fails to regenerate. There are several limitations in this study such as unknown initial tissue composition, unclear parameters in the system, and lack of molecular mechanisms. Future integration of genetic circuits and experimental validation will enhance model accuracy and clinical relevance. Ultimately, this work suggests that personalized simulations can support diagnosis of certain disease and intervention for age-related lung diseases like IPF.
Webpage: https://www.linkedin.com/in/jiho-park-computational-biologist/
